PCB 5065.
Recombination Lecture 9: Site-specific recombination systems
Instructor: Dean Gabriel
Mutagenic tools.
Homologous recombination is not only used by the cell in meiosis and mitosis;
as you will recall, it was found to be the
basis for integration of plasmid DNAs carrying yeast genes into the yeast
chromosome. Integration is frequently used for purposes of site-directed
mutagenesis, one of the main tools of functional genomics. Two of the
principal methods used for site directed mutagenesis in bacteria are marker-interruption
and marker exchange.
To perform marker-interruption, you must clone a DNA fragment that is
entirely internal to the gene that is to be the target. If either the 5'
or 3' ends of the gene are included in the clone, the interruption won't
work, for reasons that will become apparent when you diagram the event:
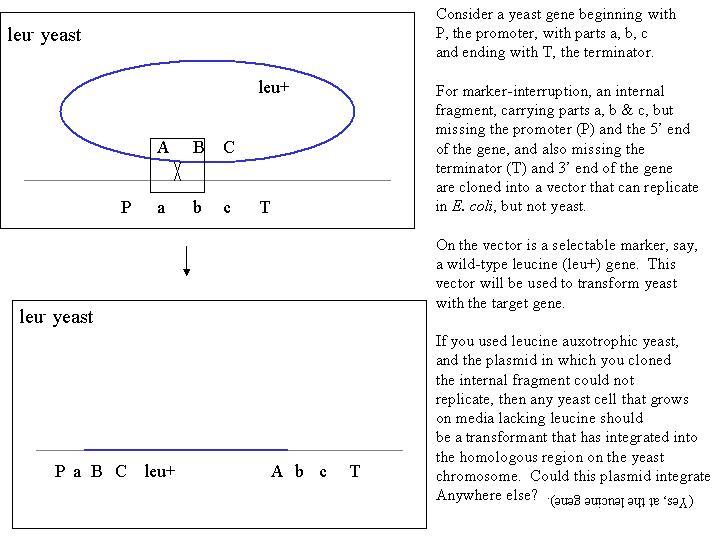
Note that the plasmid can integrate at any point that has homology, including
both the target gene, and the leucine gene.
Q: How would you ensure that the integration in yeast occurred at the
target gene only? (Hint: how was recombination
stimulated by Orr-Weaver et al? What is the mechanism of recombination???
)
Note in the above figure, there is a little black box enclosing the actual
physical exchange, which occurs by the DSBR model exactly as if the little
black box were drawn around a physical exchange region on two chromatids
in meiosis. Be sure that you can draw, using all four Watson-Crick
strands, the actual mechanism of integration, using the DSBR model. Be
sure to practice how to resolve the double Holliday structures to achieve
integration.
Prokaryotes have had to evolve a wide array of restriction endonucleases (to linearize and thus restrict double-stranded DNAs from entry),that work together with the highly efficient recBCD exonucleases (see last lecture) to protect themselves against phage infection. The recBCD exonucleases rapidly and efficiently degrade linear DNA; therefore linear DNA cannot be used to transform most prokaryotes. However, covalently closed, circular DNA can transform prokaryotes efficiently, provided the recipient strain is lacking restriction endonucleases or the covalently closed circular DNA lacks restriction endonuclease cleavage sites for those endonucleases that are produced in the recipient strain. (Phage will also produce specific methylases that protect the phage DNA from being cleaved by certain restriction enzymes.) Nevertheless, covalently closed circular DNA can even be used to transform prokaryotes by integration at reasonable efficiency, presumably because such DNA becomes (randomly) nicked, allowing binding by recA and initiating homology searching and strand invasion. Such integration requires at least 70 bp of homology in some bacteria, and much more in others, again indicating the efficiency of DNA degradation. Integration in bacteria is thought to be accomplished by double strand break repair, with the double stranded breaks resulting from paused replication forks (for more detail, refer: Lusetti, S.L. and Cox, M. M. Annual Review of Biochemistry; 2002,71:71-101),
Two methods that are widely used to make gene knockouts in bacteria depend upon reasonable efficiency of integration by covalently closed, circular plasmids that cannot replicate in the target bacteria. These methods are:
Marker Exchange
and
Splice / Overlap PCR.
Prokaryotes have also evolved a very efficient adaptive immune system, some details of which are still unknown, but which has now been coopted by researchers wishing to make both mutations as well as genome edits in eukaryotes. They all depend on Programmable Nucleases, the most common of which create Double Stranded Breaks (DSBs). These include zinc finger nucleases (ZFNs) and transcriptional activator like effector nucleases (TALENs), which rely upon protein fusions of programmable DNA binding motifs and nucleases. They also include RNA guided endoncleases based on the CRISPR system, and typically include Cas9.
Programmable nucleases and the CRISPR adaptive immune system
