Instructor: Dean Gabriel
After working through the Double Strand Break Repair (DSBR) model, try to illustrate for yourselves by drawing, how to generate 6:2s, 5:3s, ab5:3s and ab4:4s. Next ask yourself how to illustrate integration of plasmid DNA, and repair of plasmid DNA without integration (say, you were given a gapped plasmid that could replicate in yeast, using a yeast autonomously replicating sequence (ars). (Yes, they exist, and if you also provide a centromeric sequence, you can make a yeast artificial chromosome).
Now draw out a plasmid that has a very large gap in it, in a region of homology. It is a fact that gapped plasmids integrate and they never result in integration deletions. The gap is always repaired. How? Diagram this.
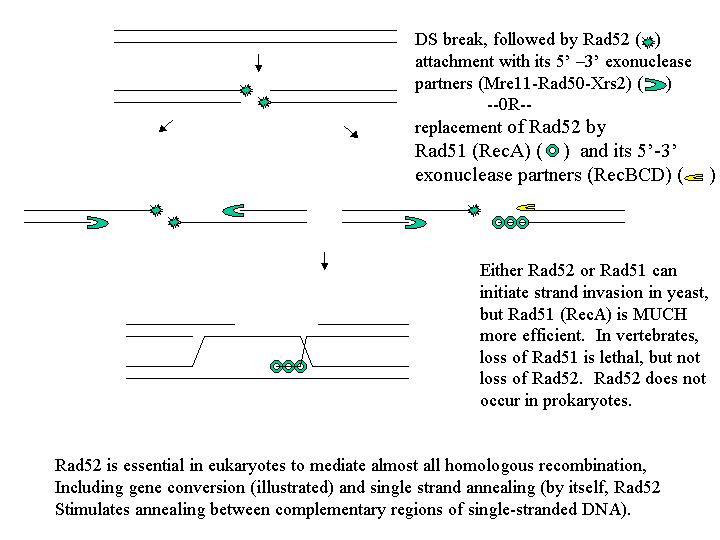
Note that the DSBR model predicts no difference in frequency between 5:3s and ab5:3s. For a long time, this fact was one of the factors that held up acceptance of the model. To make the DSBR model explain this data, one had to assume that the invading strand had a shorter resected region (single stranded region generated by 5' - 3' exonuclease) than the strand that was left to anneal with the resulting "D"-loop. However, there are now better explanations, and as you might expect, a better model.
There is some evidence now available from enzyme studies (not pure genetics, which has been used up to now to rule out or confirm the models presented so far) that there can be a basis for assuming that the 5' - 3' exonucleases involved might be different. If they are different, then it is not surprising that one might be more active than the other.

Note that the RecBCD 5’ – 3’ exonuclease activity is associated with RecA
(Rad51), while
that of Mre11-Rad50-Xrs2 is associated with Rad-52. It might be expected
that these activities
would be of different efficiencies, and could account for the lack of ab5:3
conversions expected
by the DSBR model of recombination.
In addition, very recent evidence indicates an important role for another RecA paralog, Dmc1, in meiotic crossovers in
many eukaryotes. Mutants lacking Dmc1 in several different organisms are blocked in meiotic crossing over. It has
also been proposed that Dmc1 and Rad51 may bind to opposite ends of a double stranded break, with Dmc1 controlling
strand invasion in meiosis, but this needs confirmation (Neale & Keeney 2006. Nature 442:153-157).
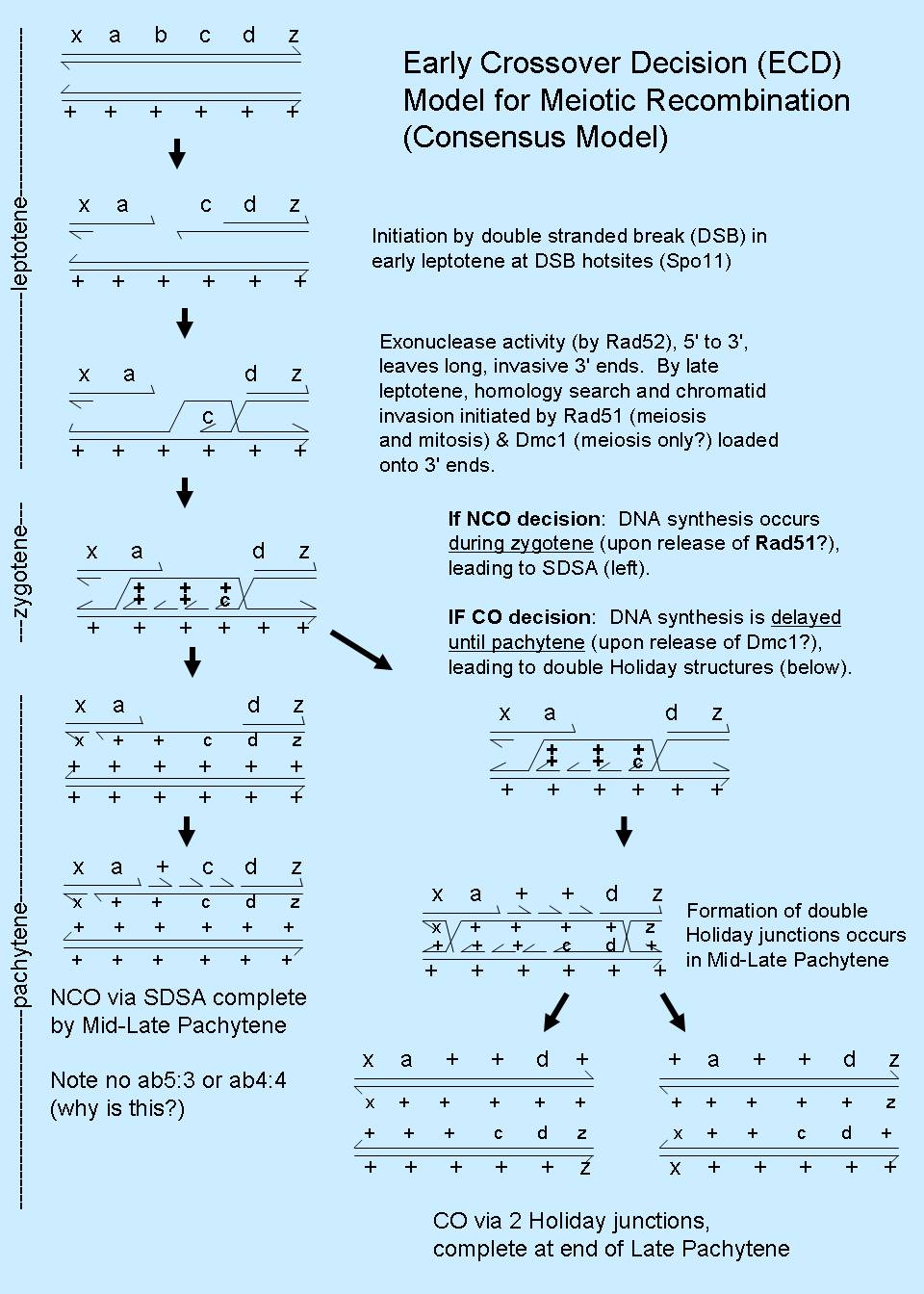
Indeed, it is now clear that DNA synthesis occurs during noncrossover (NCO) recombination in meiosis at a distinctly earlier
stage than crossover (CO) recombination, and that the decision for crossover/noncrossover is reached well before
double Holliday junction formation. In addition, although double Holliday junctions should theoretically be able to resolve with
50% CO and 50% NCO products, available evidence suggests that double Holliday junctions give rise primarily or exclusively to CO
products (Hunter & Kleckner (2001) Cell 106:59-70; Allers & Lichten (2001) Cell 106:47-57).
This has given rise to a hybrid model, called the Early Crossover Decision (ECD) model, and it is currently the
consensus model for meiotic recombination (refer Figure), although the data supporting it are primarly from budding yeast.
The noncrossover pathway is thought to occur via the synthesis dependent
strand annealing model (SDSA model), a simple model that is also thought to account for mitotic recombination. (Animated GIF
downloaded from the Sekelsky lab (http://sekelsky.bio.unc.edu/).
The SDSA pathway eliminates ab4:4 and ab5:3 tetrads, and so more obviously accounts for the genetic data than the DSBR model. The meiotic
crossover pathway is thought to occur via the DSBR model, with the restriction that double Holliday junctions resolve with crossing over.
Evidence supporting the ECD model for meiosis (combines SDSA and DSBR models, with restrictions on the resolution of DSBs)
comes primarily from mutational analyses of the helicase gene mer3, mismatch repair genes msh4 and msh5, synaptenemal complex
genes zip1, zip2, and zip3. Mutations in any of these genes reduces or eliminates meiotic crossovers (COs), but not
noncrossovers (NCOs). NCO recombinants apparently form without double Holliday junctions, and it is not yet
clear which proteins serve as determinant gatekeepers to form CO vs. NCO products in meiosis.
Evidence to support this model has now been accumulating in several labs, and enjoys considerable molecular genetics, cytological
and biochemical support. Some of this data is summarized below.
Since transformation in eukaryotes involves homology searching by already broken double-stranded DNA, how do chromosomes manage to avoid breaking apart during meiosis? The answer is far from complete. Prior to the double strand breaks, there may be a rough association of homologs that depends on telomeric interactions (Joyce and McKim 2007. BioEssays 29:217-226).
Initiation of a double strand break generates about 1 kilobase of ssDNA, and a
homology search occurs that is the equivalent of searching a 10 km long string to find a particular 20 cm long region (Neale & Keeney, 2006, Nature 442:153-8).
The homology search results in strand invasion, and if a crossover is initiated, then the formation of the synaptonemal complex is
also initiated. The synaptenemal complex brings the dyads in tight alignment with one another.
Additional studies have now conclusively shown that the synaptic
initiation sites are formed at sites of crossovers in most organisms, although there are some, notably Drosophila and Caenorhabditis elegans,
which form pairing sites and initiate synapsis in the absence of crossing over (Joyce and McKim 2007. BioEssays 29:217-226).
The following
chart from Terasawa et al. (2007) illustrates the events that occur during prophase of meiosis I. :
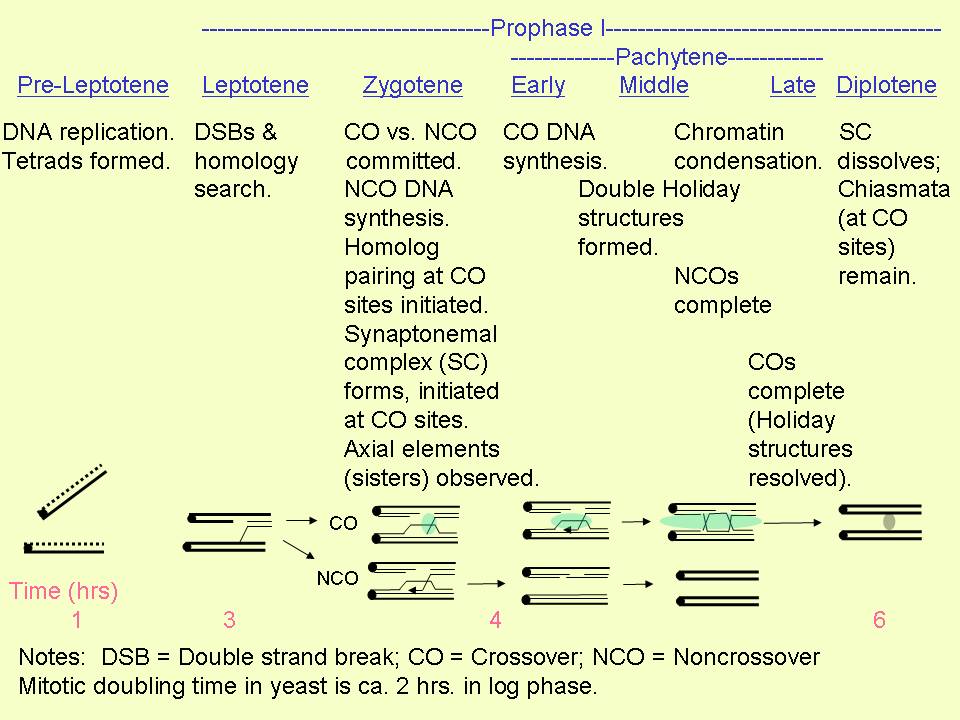
For reference, the (mitotic) doubling time of yeast is approximately
2 hrs. in log phase.
Although biochemists sometimes use the word "synapsis" to indicate a stable, homology-specific
contact, the term is used by geneticists to refer specifically to a discernable
segment of synaptenemal complex by electron microscopy or immunofluorescence
methods. (A discussion of this may be found in Zickler & Kleckner,
1999 Ann. Rev. Genet. 3:603-754). Pairing is observed to occur even in
vegetatively growing cells, and pairing involves specific sites along the
lengths of chromosomes. These sites correlate well with sites for double
strand breaks, and specifically those double strand breaks that are destined to become
crossovers.
| Organism |
# loci mapped |
1N chromosome # |
Avg. DNA length per 50 cM |
# exchanges per meiosis |
| N. crassa (bread mold) |
400 |
7 |
360 kb |
? |
| S. cerevisiae (brewer's yeast) |
175 |
17 |
250 kb |
76 |
| D. melanogaster (fruit fly) |
? |
4 |
25,000 kb |
9 |
| M. musculus (mouse) |
287 |
20 |
90,000 kb |
44 |
| Z. mays (maize) |
? |
10 |
100,000 kb |
36 |
Question for review: Why does the SDSA model (the NCO pathway in the ECD model) eliminate ab4:4s and ab5:3s???