| Bacterial Wilt of Tomato | |||
| Roll your mouse over the red-colored words to see word definitions. This will open Pop-up windows. If not, print the glossary |
|||
| Printer friendly |
PDF file |
Glossary |
|
| Symptoms & signs | Causal organism | Disease cycle & epidemiology | Diagnosis & identification | Management | References |
Bacterial wilt is one of the major diseases of tomato and other
The Solanaceae family, also known as the "nightshade" family, is a family of flowering plants, many of which are edible, while others are poisonous. The family includes the Datura or Jimson weed, eggplant, mandrake, deadly nightshade or belladonna, capsicum, potato, tobacco, tomato, and petuniasolanaceous plants. The disease is known to occur in the wet tropics, subtropics and some temperate regions of the world.
The disease is caused by the A bacterium is a microscopic organism consisting of individual cells. Bacteria cause diseases in many host plants. They can survive on crop residue, seed, or in soil and water; they may be spread by plant or plant cuttings transfer, mechanical means, insects, and seeds bacterium Ralstonia solanacearum, previously known as Pseudomonas solanacearum. It is one of the most damaging plant A pathogen, or infectious agent, is a biological agent that causes disease or illness to its host. A number of different organisms can cause plant infectious disease. Among them are fungi, bacteria, viruses, nematodes or parasitic plants pathogens. A strain is a genetic variant or subtype of a microorganism (for example virus or bacterium or fungus)Strains of this pathogen affect more than 200 plant A species is one of the basic units of biological classification. A species is often defined as a group of organisms capable of interbreeding and producing fertile offspring. Each species is placed within a single genusspecies in over 50 families throughout the world, including a wide range of crop plants, ornamentals and weeds.
Strains of R. solanacearum have conventionally been classified as A race is formed by a group of bacterial strains that are differentiated based on the response on a set of host differentialsraces and A biovar is a group of bacterial strains that are distinguishable from other strains of the same species on the basis of their physiological characteristicsbiovars (see the causal organism section for more details). Bacterial wilt of tomato is caused by either race 1 or race 3 of R. solanacearum and, rarely, by race 2. Race 1 is Endemic, in a broad sense, can mean "belonging" or "native to", "characteristic of", or "prevalent in" a particular geography, area, or environment; native to an area or scopeendemic in the United States and can cause bacterial wilt on several major crops such as eggplant, pepper, potato, tobacco and tomato. Although several introductions of race 3 to the United States have occurred as a result of importation of infected geranium cuttings from production sites off-shore, this race has been eradicated so far and is not considered established in North America. However, because of the risk of its possible re-introduction and its potential to affect potato in the northern United States, R. solanacearum race 3 biovar 2 is considered a serious threat to the United States potato industry. It is of quarantine importance and has been listed as a Select Agent plant pathogen under the Agricultural Bioterrorism Act of 2002.
| Symptoms and signs |
At the early stages of disease, the first visible symptom is a subjective evidence of disease or physical disturbance. It is an evident reaction by a plant to a pathogen, and is not necessarily visible. Different pathogens can induce similar symptomssymptoms of bacterial wilt are usually seen on the foliage of plants. These symptoms consist of wilting of the youngest leaves at the ends of the branches during the hottest part of the day (Photo 1).
At this stage, only one or half a leaflet may wilt, and plants may appear to recover at night, when the temperatures are cooler. As the disease develops under favorable conditions, the entire plant may wilt quickly and desiccate although dried leaves remain green, leading to general wilting and yellowing of foliage and eventually plant death. Another common symptom that can be associated with bacterial wilt in the field is stunting of plants (Photo 2). These symptoms may appear at any stage of plant growth, although in the field it is common for healthy-appearing plants to suddenly wilt when fruits are rapidly expanding.
 |
 |
|
| Photo 1. Symptom of bacterial wilt of tomato caused by R. solanacearum showing wilting of leaves at the end of plant branch. (Photo courtesy of Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org) |
Photo 2. Symptom of bacterial wilt of tomato caused by R. solanacearum showing wilting of foliage and stunting of plant. (Photo courtesy of C. Allen, University of Wisconsin) |
|
In young tomato stems, infected Vascular, or conductive, bundles are responsible for long-distance transport of water and nutrients throughout the plant. Highly developed plants have two types of vascular tissues: the xylem and the phloemvascular bundles may become visible as long, narrow, dark brown streaks. In young, succulent plants of highly susceptible varieties, collapse of the stem may also be observed (Photo 3). |
 |
Photo 3. Symptom of bacterial wilt of tomato caused by R. solanacearum |
In well-established infections, cross-sections of stems may reveal brown discoloration of infected tissues (Photo 4). |
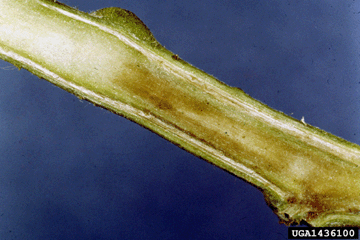 |
Photo 4. Brown discoloration of stem tissues caused by R. solanacearum. |
Symptom expression is favored by high temperatures (29-35ºC85-95ºF) and symptoms of the disease may progress rapidly after infection. However, under favorable conditions, symptomless plants may remain latently infected for extended periods of time. After infection the pathogen may survive in and be spread from the infected plant.
A common sign of bacterial wilt of tomato observed at the surface of freshly-cut sections from severely infected stems is a sticky, milky-white exudate, which indicates the presence of dense masses of bacterial cells in infected vascular bundles, and particularly in the The xylem is responsible for transportation of raw sap (water and nutrients) from roots to aerial parts of the plant. R. solanacearum is a limited xylem-invading pathogenxylem (Photo 5). |
 |
Photo 5. Bacterial ooze from freshly-cut section of a geranium stem |
Another common sign of the disease can be observed when the cut stem sections are placed in clear water as shown in Photo 6. It consists of a viscous white spontaneous slime streaming from the cut end of the stem. This streaming represents the bacterial ooze exuding from the cut ends of colonized vascular bundles (Photo 6). |
 |
Photo 6. Bacterial streaming in clear water from stem cross-section |
| Causal organism |
Ralstonia solanacearum (Smith 1896) Yabuuchi et al. 1996, is a
Bacteria have been classified as Gram-negative or Gram-positive regarding structural differences in their cell walls. Many species of Gram-negative bacteria are pathogenic. This pathogenic capability is usually associated with certain components of Gram-negative cell wallsGram-negative , rod-shaped, strictly
An aerobic organism requires oxygen for aerobic cellular respiration. Cellular respiration is the mechanism by which aerobic organism require oxygen to utilize substrates (for example sugars and fats) in order to obtain energyaerobic bacterium that is 0.5-0.7 x 1.5-2.0 µm in size. It is very sensitive to desiccation and is inhibited in culture by low concentrations (2%) of sodium chloride (NaCl). For most strains, the optimal growth temperature is
28-32ºC82-90ºF; however some strains have a lower optimal temperature of
27ºC80.5ºF.
Liquid and solid (agar) growth media are commonly used for culture of the bacterium. On solid agar medium, individual
On the surface of a solid growth medium, individual bacterial cells will grow and multiply to become visible bacterial colonies. All cells within the colony descend from a single ancestor and are identical. Characteristics of bacterial colonies (color, aspect, diameter or growth rate) are commonly used for bacteria identificationbacterial colonies are usually visible after 36 to 48 hours of growth at
28ºC82.4ºF, and two main colony types differing in morphology can be distinguished: colonies of the normal or
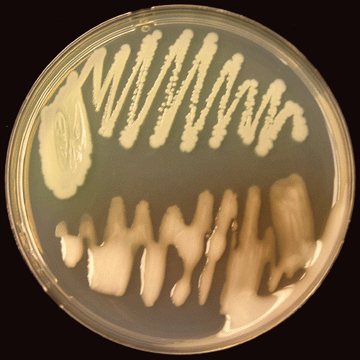
Virulence refers to the degree of pathogenicity of a microorganism, or in other words the relative ability of a microorganism to cause diseasevirulent type that are white or cream-colored, irregularly-round, fluidal, and opaque; and colonies of the mutant or non-virulent type that are uniformly round, smaller, and butyrous (dry) (Photo 8).
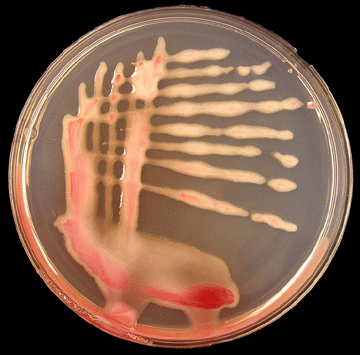
This shift from virulent to non-virulent bacterial cells occurs during storage or under oxygen stress in liquid media. A tetrazolium chloride (TZC) medium was developed to differentiate between the two colony types, in which virulent colonies appear white with pink centers and non-virulent colonies appear dark red (Photo 9). A semi-A growth or culture medium is a substance in which microorganisms, such as bacteria, or cells can grow. Selective media are used for the growth of only select microorganisms. They usually contain antibiotics to which the select microorganims is resistant toselective medium, called modified SMSA medium, has been developed for detection of R. solanacearum in water and soil samples, and in plant extracts. On this medium, typical bacterial colonies appear fluidal, irregular in shape, and white with pink centers after 2 to 5 days incubation at 82.4ºF (see the detection and identification section).
 |
 |
|
| Photo 8. Virulent (bottom) and non-virulent (top) colonies of R. solanacearum on CPG agar growth medium. (Photo courtesy of P. Champoiseau, University of Florida) |
Photo 9. Virulent colonies of R. solanacearum on TZC agar medium. (Photo courtsey of P. Champoiseau, University of Florida) |
|
For long term culture storage, R. solanacearum will remain viable for several years at room temperature in sterilized tap, distilled or deionized water.
R. solanacearum is widespread in the tropics and subtropics around the world and many strains of the pathogen have been identified and characterized so far, revealing significant
here refers to variation of a given characteristic from one bacterial strain (or group of strains) to the othervariability within the species. R. solanacearum is therefore considered a “R. solanacearum is considered a "species complex" as it includes individual isolates that may not be considered within a single species, as it is the case for the banana blood disease bacterium or Pseudomonas syzygiispecies complex”. Based on variability in host range and in ability to utilize several
Carbohydrates are simple organic compounds such as sugars and starch which contain carbon chains. They fill numerous roles in living organisms, such as the storage and transport of energy and structural components. Carbohydrates are differentially used as source of energy by bacteriacarbohydrate substrates, R. solanacearum strains were initially subdivided into races and biovars. So far, five races and five biovars have been identified within the species, but this old classification system is unsatisfactory because it is not predictive and some groups (e.g. race 1) contain very large variation. Recently, a new classification scheme has been described for strains of R. solanacearum, based on
Comparison of DNA sequences is commonly used for classification studies of strains of microorganisms. It is basically assumed that the higher the homology is between two strains, the more closely related the strains are in terms of evolution. These types of studies are known as phylogenetic studiesvariation of DNA sequences. Four
A phylotype is defined as a group of strains that are closely related based on phylogenetic analysis of sequence data. Each phylotype is composed of a number of sequevarsphylotypes were identified within the species that broadly reflect the ancestral relationships and geographical origin of the strains.
These phylotypes can be further subdivided into
A sequevar, or sequence variant, is defined as a group of strains with a highly conserved sequence within the area sequencedsequevars.
Both race 1 and race 3 can cause bacterial wilt of tomato with similar disease symptoms. Race 1 corresponds to biovars 1, 3, and 4. It has a broad host range and contains strains that infect many other major economic crops and ornamentals worldwide, such as banana, eggplant, geranium, peanut, pepper, potato, tobacco and tomato. This race is limited to tropical, subtropical and warm-temperate locations and usually cannot survive under cool temperate conditions. Race 3, which strictly corresponds to biovar 2 (or 2-A), has a limited host range. Initially described as
Pathogenicity is the ability of an organism to cause disease in another organismpathogenic on potato and tomato, it was shown to infect and induce symptoms on eggplant, geranium, and pepper. Other solanaceous and non solanaceous weeds, such as the bittersweet or woody nightshade (Solanum dulcamara) (Photo 9), are considered as alternate hosts. Most of these alternate hosts remain latently infected and may not show any disease symptoms, but they can be epidemiologically important as inoculum sources and refuges.
 |
Photo 9. Bittersweet or woody nightshade (Solanum dulcamara). |
Sometimes referred to as the ‘cold tolerant’ race, R. solanacearum race 3 biovar 2 originated in the Andes and was probably disseminated worldwide on potato tubers. It is now known to occur in the highlands of the tropics and in subtropical and temperate areas throughout the world, except in North America. In Europe, it has been responsible for several outbreaks of brown rot of potato during the last three decades. Race 3 biovar 2 occasionally causes serious losses on tomato, usually at higher altitudes in the tropics.
| Disease cycle and epidemiology |
R. solanacearum is a soilborne and waterborne pathogen; the bacterium can survive and disperse for various periods of time in infested soil or water, which can form a reservoir source of inoculum.
The bacterium usually infects tomato plants through the roots (through wounds or at the points of emergence of lateral roots). Soilborne organisms, such as the Root-knot nematodes are plant-parasitic roundworms from the genus Meloidogyne. They exist in soil in areas with hot climates or short winters. There are a great many parasitic forms, including pathogens in most plants, animals, and also in humans root-knot nematode can cause injury to plant roots and favor penetration of the bacterium. Plant infection can also occur through stem injuries caused by cultural practices or insect damage. In some cases, plant-to-plant spread can occur when bacteria move from roots of infected plants to roots of nearby healthy plants, often via irrigation practices. Spread of bacteria by aerial means and subsequent plant contamination through foliage is not known to occur, thus making R. solanacearum a non airborne pathogen. High temperatures
(29-35ºC)(85-95ºF) play a major role in pathogen growth and disease development. Several other factors that may affect pathogen survival in soil and water may also favor disease development, including soil type and structure, soil moisture content, organic matter in soil, water pH and salt content, and the presence of antagonist microorganisms.
The bacterium also has an “exterior” phase (epiphyte) in which it can reside on the outside of the plant. It is of minor importance in epidemiology of the pathogen since bacteria do not survive epiphytically for long periods of time when exposed to hot conditions or when relative humidity is below 95%.
Under favorable conditions, tomato plants infected with R. solanacearum may not show any disease symptoms. In this case, latently infected plants can play a major role in spread of the bacterium. In the United States, the southern states (Georgia and Florida) are a major source of tomato transplants for the north-eastern states and southern Canada and as a result bacterial wilt of tomatoes is occasionally found in the north via infected seedlings. The organism does not overwinter in the north, however. Transplants are either field-grown (not common anymore) or container-grown in greenhouses. Cultural practices at either field production (high plant density, use of irrigation several times a day, multiple clipping, or plants undercutting before harvest) or greenhouse production (overhead irrigation or plant handling) may favor plant infection and spread of the pathogen from infected tomato transplants production sites to healthy tomato growing sites.
R. solanacearum can survive for days to years in infected plant material in soils, infested surface irrigation water, and infected weeds.
From these sources of inoculum, bacteria can spread from infested to healthy fields by soil transfer on machinery, and surface runoff water after irrigation or rainfall. R. solanacearum can also be propagated in infested ponds or rivers and disseminated to non-infested fields through waterways. Infected semi-aquatic weeds may also play a major role in disseminating the pathogen by releasing bacteria from roots into irrigation waters.
At low temperatures (< 4ºC39.2ºF), bacterial population densities fall rapidly but the bacteria still can survive, often in a physiological latent state. In natural habitats, R. solanacerum race 3 biovar 2 can survive during the winter in semi-aquatic weeds, in plant debris or in the rhizophere of non-host plants that act as reservoirs for the pathogen. Bacteria were shown to be increasingly released from semi-aquatic weeds after winter when temperatures start to increase.
| Diagnosis and identification |
Symptom identification is the first step for early diagnosis of bacterial wilt of tomato. Accurate identification of R. solanacearum from either symptomatic or asymptomatic plants and from water or soil samples demands multiple microbiological and molecular methods. A battery of complementary tests that differ in their sensitivity and/or specificity should be used for field or laboratory analyses for unambiguous identification of bacteria to species and biovar.
Screening tests can facilitate early detection and identification of bacteria in potentially infected plants or contaminated soil and water samples by R. solanacearum. They cannot be used to identify the race or biovar of the organism. These screening tests include stem streaming, plating on semi-selective medium (modified SMSA),
These assays are based on the use of antibiotics in various test formats to detect and identify any molecules or cells (including bacteria). The most commonly used assays for bacteria detection and identification are agglutination, enzyme-linked immunosorbent assay (ELIZA), immunofluorescence, lateral flow strip tests or flow-through assaysimmunodiagnostic assays using R. solanacerum specific
Antibodies (also known as immunoglobulins) are proteins that are used by the immune system to identify and neutralize foreign objects, such as bacteria and viruses. Due to their specificity, they are commonly used in biology for detection and identification of microorganismsantibodies,
A nucleic acid is a molecule composed of nucleotide chains. These molecules carry genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are universal in living things, as they are found in all cells and virusesnucleic-acid-based identification using R. solanacerum specific primers, and pathogenicity assessment using susceptible hosts (e.g. tomato seedlings). Several rapid screening tests, such as immunostrips (Agdia), are available commercially for rapid and field detection of R. solanacearum.
A biochemical growth test is used for biovar determination of R. solanacearum. This test is based on the differential ability of strains of the pathogen to differentially produce acid from several carbohydrate sources, including disaccharides and sugar alcohols.
At the sub-species level, identification of strains of R. solanacearum can be assessed with several nucleic-acid based methods such as
DNA probe hybridization uses the ability of two complementary single-stranded nucleic acids to combine into a single molecule. Nucleotide probe of known sequence will be used to bind complementary strand of undetermined organism for identificationDNA probe hybridization and especially
The polymerase chain reaction is a technique that consists of amplifying a DNA molecule exponentiallypolymerase chain reaction (PCR) amplification with specific probes and primers.
Race determination is not generally possible because R. solanacearum strains usually have numerous hosts and do not have race-cultivar specificity on plant hosts. This is why the race sub-classification system has fallen out of favor with scientists, although it still has regulatory meaning because of quarantine rules written for “race 3 biovar 2”.
It is important to understand that unequivocal identification of R. solanacearum race 3 biovar 2 must rely on at least two distinct methods, including the biovar test and one of the nucleic acid-based tests that use PCR to amplify one of several specific DNA fragments.
Currently, for regulatory purposes, the only laboratory with proper registrations for ultimate determination of race and biovar of R. solanacearum race 3 biovar 2 is the USDA-APHIS-PPQ National Plant Germplasm and Biotechnology Laboratory in Beltsville, M. D.
| USDA-APHIS-PPQ-CPHST BARC-East, Bldg. 580 Powder Mill Road Beltsville, MD 20705 Phone number: 301-504-7100 Fax number: 301-504-8539 |
| Management |
Bacterial wilt of tomato is difficult to control, and no single strategy has shown 100% efficiency in control of the disease so far.
Bactericides (copper) and antibiotics (streptomycin, ampicillin, tetracycline and penicillin) have shown little efficiency on suppression of R. solanacearum in the field and are environmentally destructive and fairly expensive to apply. As a result, a combination of diverse control methods, including host resistance, cultural practices, and the use of chemical or biological control should be used in an integrated pest management approach to control bacterial wilt of tomato in locations where the pathogen is established. These methods should be used in combination for best management of bacterial wilt of tomato. Most of following recommendations are applicable to race 1 strain of R. solanacearum only.
Some level of bacterial wilt control is possible using resistant or moderately resistant tomato A cultivar is a cultivated plant that has been selected and given a unique name because it has desirable characteristics (decorative or useful) that distinguish it from otherwise similar plants of the same speciescultivars, such as FL7514 and BHN 466. However, resistant cultivars may produce fruits that are reduced in size and thus not acceptable to the commercial tomato industry. Additionally, resistance in these cultivars may vary with location and temperature, because of strain differences. Grafting susceptible tomato cultivars onto resistant tomato or other solanaceous rootstocks is effective and used on the commercial scale in Japan, Bangladesh, and the Philippines. Grafting has not been tested for use against R3bv2 and some reports suggest that bacterial wilt-resistant tomato cultivars and breeding lines may have poor resistance to R3bv2.
Chemical control by soil fumigation (chloropicrin) or application of phosphorous acid is also expensive to apply; soil fumigation has been reported to achieve limited success if combined with other control methods. When used, chemical control should be integrated with other methods to reduce selection pressure for pathogen resistance.
Biological control is defined as the reduction of pest populations (including insects, mites, weeds and plant diseases) by natural enemies. Biological control agents of plant diseases are most often referred to as antagonistsBiological control, based on use of R. solanacearum antagonists, and use of A suppressive soil is one that possesses some level of control of a disease forming organism. All soils have a natural level of disease suppressive activities. In most soils long term management can either reduce or increase this level of suppressionsuppressive soils has shown promising results at the small experimental scale, but still needs to be validated at a larger scale.
The use of Thymol, a plant-derived volatile chemical that is not commercially available yet, was shown to reduce disease incidence and increase yield in field experiments in Florida. Similarly, the application of Actigard (Syngenta), a Plant resistance inducers are natural or synthetic chemical compounds that apparently act by stimulating the natural defense response in the plantplant resistance inducer, in combination with moderately resistant cultivar was shown to enhance resistance against the disease at field scale in Florida.
Best strategy for control of bacterial wilt in the field consists primarily of phytosanitation and cultural practices. In the regions where the disease is endemic, these methods have proven to be effective in some conditions, and should be used:
Before plantation:
• Consider an effective weed control in and around tomato fields and aquatic weed control around irrigation ponds.
• Apply 3-4 years rotation and cover crops for infested fields to reduce R. solanacearum, weeds and nematodes.
• Do not irrigate rotation and cover crops with R. solanacearum contaminated pond or surface water, avoid reinfestation.
• Use well drained and leveled fields and do not use low-lying areas of the field.
• Raise soil pH to 7.5-7.6 and increase available calcium (liming).
• Consider using infested fields (after 3-4 years rotation) during cooler months for tomato production (i.e., spring season for north Florida).
During production
• Exclude the pathogen by applying strict sanitation practices (pathogen free irrigation water, transplants, stakes, machinery, etc.).
• Chlorinate your irrigation water continuously if you are using surface water or R. solanacearum infested pond water.
• Continue an effective weed control in and around tomato fields and irrigation ponds.
• Irrigate based on water need, avoid over irrigation.
• Apply plant resistance inducer, such as Actigard (Syngenta) if you are using moderately resistant cultivars (i.e., FL 7514). Actigard enhances resistance against this disease if it is used in combination with moderately resistant cultivars.
After harvest
• Plow under crop residue immediately.
• Start with suitable rotation and cover crops (i.e., rye for winter, sudan-sorghum for summer in north Florida) to avoid weeds that support R. solanacearum populations.
In areas where R. solanacearum race 3 biovar 2 is not known to be established, such as the United States, the pathogen could be carried infected plant material 9such as geranium cuttings) imported from off-shore production sites. The first strategy is to prevent introduction and, if inadvertently introduced, subsequent movement of the pathogen. This can best be achieved with strict phytosanitary and regulatory procedures.
A “New Pest Response Guidelines” (USDA-APHIS-PPQ) and a "Recovery plan for Ralstonia solanacearum race 3 biovar 2" (USDA-ARS) give the most accurate available information for detection, control, containment, and eradication of R. solanacearum race 3 biovar 2.
Exclusionary practices, such as quarantine, testing and visual inspection of imported material of host plants, regulation and establishment of minimum sanitation protocols for offshore geraniums cutting production can prevent introduction of the pathogen. Practices such as cleaning and sanitizing field and handling equipment, and application of good sanitary cultural practices will prevent movement of the pathogen from infested to pathogen-free fields in case of inadvertent introduction of the pathogen. At the greenhouse, sanitary practices for tomato transplants production may include avoidance of sub-irrigation, wide separation of greenhouses from field production areas, disinfection of all frames, trays and tools, use of pathogen-free soils or potting mix, control of weeds, and limited handling of plants.
Along with the establishment of exclusionary strategies, it is critical to monitor potentially infected sites for early detection and further eradication of R. solanacearum race 3 biovar 2. Potentially infected sites to be monitored include soils in which crops infected by the pathogen have been identified, rivers and other surface water used for irrigation, particularly when infected host weeds are present.
Because R. solanacearum race 3 biovar 2 is on the Select Agent list in the United States, detection and confirmation of this pathogen by a USDA-APHIS-PPQ recognized authority involves the establishment of a set of protocols and measures to deal with potential outbreaks of the disease. APHIS, in cooperation with the state(s), is responsible for implementing these measures. Immediate reaction might take place in the form of teams of experts and survey personnel being sent to the site of initial detection in order to place holds, conduct investigations, and initiate surveys. Further response activities which may be taken include implementing regulatory measures to quarantine infected or potentially infected production areas, stopping the intrastate and interstate movement of infected or potentially infected articles in commerce, and identifying proper control measures, which may include host removal and destruction, or requiring sanitary practices to eradicate the pathogen.
Confirmed infestations of tomato or other solanaceous crops by R. solanacearum race 3 biovar 2 will require quarantine of fields, tomato transplants, seedlings, or other plant material associated with infested lots, including processing facilities, storage bins, means of conveyance, soil, and irrigation water. Host removal and destruction is required along with disinfection, as well as several years of non-host production in infested fields or associated growing areas before the quarantine can be removed. In case of contamination of water by the pathogen, irrigation with surface water should be prohibited, and water treatments, such as filtration or chemical disinfection, may be applied under control of legal authorities. Any permission to irrigate would be subject to results from sampling and testing water samples.
| References |
CABI/EPPO. 1999. Distribution maps of plant diseases. Map N0. 785 CAB International. Wallingford, U. K.
Boshou, L. 2005. A broad review and perspective on breeding for resistance to bacterial wilt, p. 225-238. In C. Allen, P. Prior, and A. C. Hayward (ed.), Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex. APS Press, St. Paul, MN.
Coutinho, T. A. 2005. Introduction and prospectus on the survival of R. solanacearum. Pages 29-38 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. Allen, C., Prior, P., and Hayward, A. C., eds. APS press, St. Paul, M. N.
Denny, T. P. 2006. Plant pathogenic Ralstonia species. Pages 573-644 in: Plant-associated bacteria. S. S. Gnanamanickam, ed. Springer Publishing, Dordrecht, The Netherlands.
Elphinstone, J. G. 2005. The current bacterial wilt situation: a global overview. Pages 9-28 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. C. Allen, P. Prior, and A. C. Hayward, eds. APS press, St-Paul, M. N.
Fegan M. and Prior P. 2005. How complex is the "Ralstonia solanacearum" complex ? Pages 449-461 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. C., Allen, P., Prior, A. C., Hayward, eds. APS Press, St. Paul, M. N.
Gitaitis, R., McCarter, S., and Jones, J. 1992. Disease control in tomato transplants produced in Georgia and Florida. Plant Disease 76:651-656.
Ji, P., M. T. Momol, S. M. Olson, and P. M. Pradhanang. 2005. Evaluation of thymol as biofumigant for control of bacterial wilt of tomato under field conditions. Plant Disease 89:497-500.
Kucharek, T. 1998. Bacterial wilt of row crops in Florida. 9 p. University of Florida, Cooperative Extension Service, Institute of Food and Agricultural Sciences, Florida, U. S. Cir. 1207.
Lambert, C. D. 2002. Agricultural bioterrorism protection act of 2002: possession, use, and transfer of biological; agents and toxins; interim and final rule. (7 CFR Part 331). Federal Register 67:76908-76938.
Pradhanang, P.M., Ji, P., Momol, M.T., Olson, S.M., Mayfield, J.L., and Jones, J.B. 2005. Application of acibenzolar-S-methyl enhances host resistance in tomato against Ralstonia solanacearum. Plant Disease 89:989-993.
McCarter, S. M. 1991. Bacterial wilt. Pages 28-29 in: Compendium of tomato diseases. Jones, J. B., Jones, J. P., Stall, R. E., and Zitter, T. A., eds. APS Press Publisher: St. Paul, M. N.
Saddler, G. S. 2005. Management of bacterial wilt disease. Pages 121-132 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. Allen, C., Prior, P., and Hayward, A. C., eds. APS press, St. Paul, M. N.
| Author: | Patrice G. Champoiseau and Timur M. Momol of University of Florida |
| Reviewers: | Caitilyn Allen of University of Wisconsin; Jeffrey B. Jones, and Carrie Harmon of University of Florida |
| Publication date: | September 12, 2008. Revised April 04, 2009 |
| Supported by: | The United States Department of Agriculture - National Research Initiative Program (2007-2010) |

