| Southern Wilt of Geranium | |||
| Roll your mouse over the red-colored words to see word definitions. This will open Pop-up windows. If not, print the glossary |
|||
| Printer friendly |
PDF file |
Glossary |
|
| Symptoms & signs | Causal organism | Disease cycle & epidemiology | Diagnosis & identification | Management | References |
Southern wilt, also known as bacterial wilt, is currently an important disease for geranium producers. It is caused by the A bacterium is a microscopic organism consisting of individual cells. Bacteria cause diseases in many host plants. They can survive on crop residue, seed, or in soil and water; they may be spread by plant or plant cuttings transfer, mechanical means, insects, and seeds bacterium Ralstonia solanacearum, previously known as Pseudomonas solanacearum. R. solanacearum It is one of the most damaging plant A pathogen, or infectious agent, is a biological agent that causes disease or illness to its host. A number of different organisms can cause plant infectious disease. Among them are fungi, bacteria, viruses, nematodes or parasitic plants pathogens worldwide. A strain is a genetic variant or subtype of a microorganism (for example virus or bacterium or fungus)Strains of this pathogen affect more than 200 plant A species is one of the basic units of biological classification. A species is often defined as a group of organisms capable of interbreeding and producing fertile offspring. Each species is placed within a single genusspecies in over 50 families throughout the world, including a wide range of crop plants, ornamentals and weeds. The pathogen is known to occur in the wet tropics, subtropics and some temperate regions of the world.
Strains of R. solanacearum have conventionally been classified as A race is formed by a group of bacterial strains that are differentiated based on the response on a set of host differentialsraces and A biovar is a group of bacterial strains that are distinguishable from other strains of the same species on the basis of their physiological characteristicsbiovars (see the causal organism section for more details). Southern wilt of geranium is caused by either race 1 or race 3 of R. solanacearum. In the United States, race 1 is Endemic, in a broad sense, can mean "belonging" or "native to", "characteristic of", or "prevalent in" a particular geography, area, or environment; native to an area or scopeendemic and can cause bacterial wilt on several major crops such as eggplant, pepper, potato, tobacco and tomato.
As a key ornamental plant, geranium are grown from
Plant cutting is a technique for vegetatively (asexually) propagating plants in which a piece of the source plant is used for obtaining a new plant. The cutting produces new roots, stems, or both, and thus becomes a new plant independent of the parentcuttings about 100 millions of which are imported each year in the United States. Geranium cuttings are produced at off-shore production facilities in geographical locations like tropical highlands where R. solanacearum race 3 is endemic. Although several introductions of race 3 in the United States have occurred as a result of importation of infected geranium cuttings, this race has been eradicated so far and is not considered to be established in North America. However, because of the risk of its possible re-introduction, and its potential to affect potato in the northern United States, R. solanacearum race 3 biovar 2 is considered a serious threat to the United States potato industry. It is of quarantine importance and has been listed as a Select Agent plant pathogen under the Agricultural Bioterrorism Act of 2002.
| Symptoms and signs |
symptom is a subjective evidence of disease or physical disturbance. It is an evident reaction by a plant to a pathogen, and is not necessarily visible. Different pathogens can induce similar symptomsSymptoms of Southern wilt of geranium usually begin with abnormal
Chlorosis is a condition in which leaves produce insufficient chlorophyll. As chlorophyll is responsible for the green color of leaves, chlorotic leaves are pale, yellow, or yellow-whitechlorosis and wilting of the lower leaves. In some cases, leaves may also show abnormal upward curling at their margins which is very characteristic of the disease (Photo 1).
At this stage, plants may appear to recover at night when the temperatures are cooler. Under favorable conditions, the disease develops rapidly and wilting may spread up the plant from older leaves to newer ones. Wilted leaves often become chlorotic then brown
In plant biology, necrosis is the name given to death of plant cells and plant tissue. The tissue first turns brown and subsequently diesnecrotic in wedge-shaped patterns that expand towards the leaf margins. The leaf margins themselves may also become chlorotic, then necrotic, and the whole plant may desiccate and die (Photo 2).
 |
 |
|
| Photo 1. Initial symptoms of Southern wilt of geranium caused by R. solanacearum showing wilting and upward curling of leaves. (Photo courtesy of D. Norman, Mid-Florida Research and Education Center, IFAS, University of Florida) |
Photo 2. Symptoms of Southern wilt of geranium caused by R. solanacearum race 3 biovar 2 showing drying and brown necrosis on leaves. (Photo courtesy of the Wisconsin Department of Agriculture, Trade and Consumer Protection) |
|
In late stages of disease, collapse of the stem may also be observed (Photo 3). Stems and roots may show brown vascular discoloration, blacken and eventually become necrotic (Photo 3, photo 4).
 |
 |
|
| Photo 3. Late symptoms of Southern wilt of geranium caused by R. solanacearum race 3 biovar 2 showing blackening and collapse of stem. (Photo courtesy of D. Norman, Mid-Florida Research and Education Center, IFAS, University of Florida) |
Photo 4. Late symptoms of Southern wilt of geranium caused by R. solanacearum showing root blackening. (Photo courtesy of Margery Daughtrey, Cornell University) |
Symptom expression is favored by high temperatures (29-35ºC85-95ºF) and symptoms of the disease may progress rapidly after infection. However, under favorable conditions, symptomless plants or their cuttings may remain latently infected for extended periods of time. After infection the pathogen may survive in and be spread from the infected plant.
Similar wilt symptoms may be caused by general root rot, various nutrient imbalances, water stress, or other bacterial pathogens, such as Xanthomonas campestris pathovar pelargonii causing bacterial blight. Generally, leaves of geranium affected with bacterial blight will be spotted, the roots will remain white and healthy, and vascular discoloration will be less pronounced or absent (see comparison of bacterial blight and Southern wilt symptoms on geranium).
A common sign of Southern wilt of geranium observed at the surface of freshly-cut sections from severely infected stems is a sticky, milky-white exudate, which indicates the presence of dense masses of bacterial cells in infected Vascular, or conductive, bundles are responsible for long-distance transport of water and nutrients throughout the plant. Highly developed plants have two types of vascular tissues: the xylem and the phloemvascular bundles, and particularly in the The xylem is responsible for transportation of raw sap (water and nutrients) from roots to aerial parts of the plant. R. solanacearum is a limited xylem-invading pathogenxylem (Photo 5). |
 |
Photo 5. Bacterial ooze from freshly-cut section of a geranium stem |
Another common sign of the disease is observed when the stem cut sections are placed in clear water as shown in Photo 6. It consists of a viscous white spontaneous slime streaming from the cut end of the stem. This streaming represents the bacterial ooze exuding from the cut ends of colonized vascular bundles (Photo 6). |
 |
Photo 6. Bacterial streaming in clear water from stem cross-section |
| Causal organism |
Ralstonia solanacearum (Smith 1896) Yabuuchi et al. 1996, is a
Bacteria have been classified as Gram-negative or Gram-positive regarding structural differences in their cell walls. Many species of Gram-negative bacteria are pathogenic. This pathogenic capability is usually associated with certain components of Gram-negative cell wallsGram-negative , rod-shaped, strictly
An aerobic organism requires oxygen for aerobic cellular respiration. Cellular respiration is the mechanism by which aerobic organism require oxygen to utilize substrates (for example sugars and fats) in order to obtain energyaerobic bacterium that is 0.5-0.7 x 1.5-2.0 µm in size. It is very sensitive to desiccation and is inhibited in culture by low concentrations (2%) of sodium chloride (NaCl). For most strains, the optimal growth temperature is
28-32ºC82-90ºF; however some strains have a lower optimal growth temperature of
27ºC80.5ºF.
Liquid and solid (agar) growth media are commonly used for culture of the bacterium. On solid agar medium, individual
On the surface of a solid growth medium, individual bacterial cells will grow and multiply to become visible bacterial colonies. All cells within the colony descend from a single ancestor and are identical. Characteristics of bacterial colonies (color, aspect, diameter or growth rate) are commonly used for bacteria identificationbacterial colonies are usually visible after 36 to 48 hours of growth at
28ºC82.4ºF, and two main colony types differing in morphology can be distinguished: colonies of the normal or
Virulence refers to the degree of pathogenicity of a microorganism, or in other words the relative ability of a microorganism to cause diseasevirulent type that are white or cream-colored, irregularly-round, fluidal, and opaque; and colonies of the mutant or non-virulent type that are uniformly round, smaller, and butyrous (dry) (Photo 7).
This shift from virulent to non-virulent bacterial cells occurs during storage or under oxygen stress in liquid media. A tetrazolium chloride (TZC) medium was developed to differentiate between the two colony types, in which virulent colonies appear white with pink centers and non-virulent colonies appear dark red (Photo 8). A semi-A growth or culture medium is a substance in which microorganisms, such as bacteria, or cells can grow. Selective media are used for the growth of only select microorganisms. They usually contain antibiotics to which the select microorganims is resistant toselective medium, called modified SMSA medium, has been developed for detection of R. solanacearum in water and soil samples, and in plant extracts. On this medium, typical bacterial colonies appear fluidal, irregular in shape, and white with pink centers after 2 to 5 days incubation at 82.4ºF (see the detection and identification section).
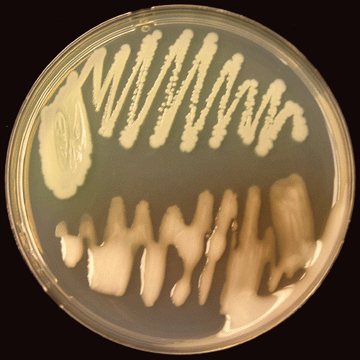 |
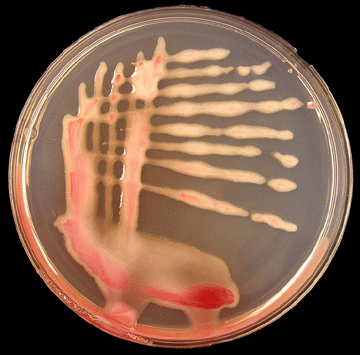 |
|
| Photo 8. Virulent (bottom) and non-virulent (top) colonies of R. solanacearum on CPG agar growth medium. (Photo courtesy of P. Champoiseau, University of Florida) |
Photo 9. Virulent colonies of R. solanacearum on TZC agar medium. (Photo courtsey of P. Champoiseau, University of Florida) |
|
For long term storage, R. solanacearum can be stored for several years at room temperature in sterilized tap, distilled or deionized water.
R. solanacearum is widespread in the tropics and subtropics around the world and many strains of the pathogen have been identified and characterized so far, revealing significant Variability here refers to variation of a given characteristic from one bacterial strain (or group of strains) to the othervariability within the species. R. solanacearum is therefore considered a “R. solanacearum is considered a "species complex" as it includes individual isolates that may not be considered within a single species, as it is the case for the banana blood disease bacterium or Pseudomonas syzygiispecies complex”. Based on variability in host range and in ability to utilize several Carbohydrates are simple organic compounds such as sugars and starch which contain carbon chains. They fill numerous roles in living organisms, such as the storage and transport of energy and structural components. Carbohydrates are differentially used as source of energy by bacteriacarbohydrate substrates, R. solanacearum strains were initially subdivided into races and biovars. So far, five races and five biovars have been identified within the species, but this old classification system is unsatisfactory because it is not predictive and some groups (e.g. race 1) contain very large variation. Recently, a new classification scheme has been described for strains of R. solanacearum, based on Comparison of DNA sequences is commonly used for classification studies of strains of microorganisms. It is basically assumed that the higher the homology is between two strains, the more closely related the strains are in terms of evolution. These types of studies are known as phylogenetic studiesvariation of DNA sequences. Four A phylotype is defined as a group of strains that are closely related based on phylogenetic analysis of sequence data. Each phylotype is composed of a number of sequevarsphylotypes were identified within the species that broadly reflect the ancestral relationships and geographical origin of the strains.
These phylotypes can be further subdivided into A sequevar, or sequence variant, is defined as a group of strains with a highly conserved sequence within the area sequencedsequevars.
Both race 1 and race 3 can cause Southern wilt of geranium with similar disease symptoms. Race 1 corresponds to biovars 1, 3, and 4 and includes members of all four phylotypes. These strains have a broad host range and may infect many other major economic crops and ornamentals worldwide, such as banana, eggplant, geranium, peanut, pepper, potato, tobacco and tomato. "Race 1" strains are limited to tropical, subtropical and warm-temperate locations and usually cannot survive under cool temperate conditions. In contrast, race 3, which strictly corresponds to biovar 2 (or 2-A), has a limited host range. Initially described as Pathogenicity is the ability of an organism to cause disease in another organismpathogenic on potato and tomato, it was shown to infect and induce symptoms on eggplant, geranium, and pepper. Other
The Solanaceae family, also known as the "nightshade" family, is a family of flowering plants, many of which are edible, while others are poisonous. The family includes the Datura or Jimson weed, eggplant, mandrake, deadly nightshade or belladonna, capsicum, potato, tobacco, tomato, and petuniasolanaceous and non solanaceous weeds, such as the bittersweet or woody nightshade (Solanum dulcamara) (Photo 10), are considered as alternate hosts. Most of these alternate hosts remain latently infected and may not show any disease symptoms, but they can be epidemiologically important as inoculum sources and refuges.
 |
Photo 10. Bittersweet or woody nightshade (Solanum dulcamara). |
Sometimes referred to as the ‘cold tolerant’ race, R. solanacearum race 3 biovar 2 originated in the Andes and was probably disseminated worldwide on potato tubers. It is now known to occur in the highlands of the tropics and in subtropical and temperate areas throughout the world, except in North America. In Europe, it has been introduced on geranium cuttings produced in the African highlands and has been responsible for several outbreaks of brown rot of potato during the last three decades.
| Disease cycle and epidemiology |
R. solanacearum is a soilborne and waterborne pathogen; the bacterium can survive and disperse for various periods of time in infested soil or water, which can form a reservoir source of inoculum. The pathogen can also survive in asymptomatic geranium cuttings obtained from infected plants. High temperature and high soil moisture usually favor survival of the pathogen.
Plant infection usually occurs when plants are grown in infested soil or irrigated with contaminated water. R. solanacearum usually infects plant through the roots (through wounds or at the points of emergence of lateral roots). Plant infestation can also occur through stem injuries caused by cultural practices, such as cutting harvest, or insect damage. Plant-to-plant spread can occur when bacteria move from roots of infected plants to roots of nearby healthy plants, often via irrigation practices. Spread of bacteria by aerial means and subsequent plant contamination is not known to occur, thus making R. solanacearum a non airborne pathogen. After penetrating the roots, the bacteria will spread into the host through vascular tissues, leading to symptom development and rapid plant death under favorable conditions. Frequently, infected geranium plants will not show any disease symptoms. Such latently infected plants can be responsible for long-distance spread of the pathogen if used for cutting production. R. solanacearum can also be spread through contaminated irrigation water, on equipment, by personnel or by soil transfer.
The bacterium also has an “exterior” phase (epiphyte) in which it can reside on the outside of the plant. It is of minor importance in epidemiology of the pathogen since bacteria do not survive epiphytically for long periods of time when exposed to hot conditions or when relative humidity is below 95%.
In geranium production facilities, the major risk for spread of R. solanacearum is through
Ebb-and-flow subirrigation is a method of irrigation that is usually used for potted plants in greenhouses. Water is delivered to the plant root zone from below the soil surface and absorbed upwardsebb-and-flow subirrigation systems, which are strongly discouraged. Infected weed species that live near irrigation water sources may also serve as source of inoculum. If an infected plant is pruned with tools or pinched by hand, the bacterium can also be spread to subsequent healthy plants. The pathogen also can be spread by splashing irrigation water. The pathogen does not readily spread through leaf-to-leaf contact or through water vapor in the air.
| Diagnosis and identification |
Symptom identification is the first step for early diagnosis of brown rot of potato. Accurate identification of R. solanacearum from either symptomatic or asymptomatic plants and from water or soil samples demands multiple microbiological and molecular methods. A battery of complementary tests that differ in their sensitivity and/or specificity should be used for field or laboratory analyses for unambiguous identification of bacteria to species and biovar.
Screening tests can facilitate early detection and identification of bacteria in potentially infected plants or contaminated soil and water samples by R. solanacearum. They cannot be used to identify the race or biovar of the organism. These screening tests include stem streaming, plating on semi-selective medium (modified SMSA),
These assays are based on the use of antibiotics in various test formats to detect and identify any molecules or cells (including bacteria). The most commonly used assays for bacteria detection and identification are agglutination, enzyme-linked immunosorbent assay (ELIZA), immunofluorescence, lateral flow strip tests or flow-through assaysimmunodiagnostic assays using R. solanacerum specific
Antibodies (also known as immunoglobulins) are proteins that are used by the immune system to identify and neutralize foreign objects, such as bacteria and viruses. Due to their specificity, they are commonly used in biology for detection and identification of microorganismsantibodies,
A nucleic acid is a molecule composed of nucleotide chains. These molecules carry genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are universal in living things, as they are found in all cells and virusesnucleic-acid-based identification using R. solanacerum specific primers, and pathogenicity assessment using susceptible hosts (e.g. tomato seedlings). Several rapid screening tests, such as immunostrips (Agdia), are available commercially for rapid and field detection of R. solanacearum.
A biochemical growth test is used for biovar determination of R. solanacearum. This test is based on the differential ability of strains of the pathogen to differentially produce acid from several carbohydrate sources, including disaccharides and sugar alcohols.
At the sub-species level, identification of strains of R. solanacearum can be assessed with several nucleic-acid based methods such as
DNA probe hybridization uses the ability of two complementary single-stranded nucleic acids to combine into a single molecule. Nucleotide probe of known sequence will be used to bind complementary strand of undetermined organism for identificationDNA probe hybridization and especially
The polymerase chain reaction is a technique that consists of amplifying a DNA molecule exponentiallypolymerase chain reaction (PCR) amplification with specific probes and primers.
Race determination is not generally possible because R. solanacearum strains usually have numerous hosts and do not have race-cultivar specificity on plant hosts. This is why the race sub-classification system has fallen out of favor with scientists, although it still has regulatory meaning because of quarantine rules written for “race 3 biovar 2”.
It is important to understand that unequivocal identification of R. solanacearum race 3 biovar 2 must rely on at least two distinct methods, including the biovar test and one of the nucleic acid-based tests that use PCR to amplify one of several specific DNA fragments.
Currently, for regulatory purposes, the only laboratory with proper registrations for ultimate determination of race and biovar of R. solanacearum race 3 biovar 2 is the USDA-APHIS-PPQ National Plant Germplasm and Biotechnology Laboratory in Beltsville, M. D.
| USDA-APHIS-PPQ-CPHST BARC-East, Bldg. 580 Powder Mill Road Beltsville, MD 20705 Phone number: 301-504-7100 Fax number: 301-504-8539 |
| Management |
Management of Southern wilt of geranium is best achieved with rigorous exclusionary practices, such as strict quarantine and sanitation procedures. In the United States, the zero-tolerance for R. solanacearum race 3 biovar 2 as a Select Agent included the reinforcement of regulations, sanitation protocols and controls to first prevent production of infected geranium cuttings off-shore, and then prevent introduction and spread from nursery facilities in the country.
A “New Pest Response Guidelines” (USDA-APHIS-PPQ) and a "Recovery plan for Ralstonia solanacearum race 3 biovar 2" (USDA-ARS) give the most accurate available information for detection, control, containment, and eradication of R. solanacearum race 3 biovar 2.
A certification program for geraniums produced outside the United States was also implemented to require specific phytosanitary practices and regular testing at facilities exporting to the United States. Geranium plants exported to the United States require phytosanitary certification and a statement that geraniums shipped to the United States are free of the pathogen.
Growers may develop and implement effective sanitation procedures to prevent pathogen introduction and ensure that R. solanacearum race 3 biovar 2 does not spread within their greenhouse or nursery facilities, associated support buildings, equipment or vehicles. A “Minimum sanitation protocols for offshore geraniums cutting production” document was developed by APHIS-PPQ in 2004 to be used by off-shore geranium suppliers. It defines minimum standards and requirements for greenhouse structure and material.
In case of possible infection of plants by R. solanacearum race 3 biovar 2 in the U.S., growers are required to immediately notify to Federal and state regulatory officers. They must conduct inspections and apply control measures to ensure that the disease or the pathogen does not spread within or between greenhouses or nurseries, associated support buildings, equipment, vehicles, or fields and does not escape other production systems. Regulatory officers are required to follow the sanitation guideline procedures before and during the survey to avoid inadvertent spread of R. solanacearum race 3 biovar 2. Surveys might be achieved by visual inspection of symptoms and signs of the disease and early detection of the pathogen by the use of rapid screening tests, such as the stem-streaming test or commercial serological quick tests.
In case of suspect contamination, recommendations of the “New Pest Response Guidelines” must be applied. Among immediate actions that must take place are the holding (no sales or movement permitted) of potentially infected plants and all plant material and equipment that may have come in contact with potentially infected plants, conduct survey to determine origin of infection, registrations and sampling procedures for further confirmation of the species of the pathogen by an approved laboratory. Samples should preferably be the whole plant, including roots without the soil if possible. Alternatively, a 2-to-3 cm long stem portion can be sampled in the lower part of the stem, above soil level, where the pathogen is concentrated. Before any testing, nursery growers have the option to destroy potentially infected plant material on hold under prescribed conditions, or maintaining the held plant material in conditions (temperature and time) that favor symptom development for disease diagnostic. If infection by R. solanacearum is confirmed by a State laboratory, the sample or culture must be sent as soon as possible for confirmation to race and biovar. The USDA-APHIS-PPQ-CPHST laboratory in Beltsville, MD, is the only diagnostic laboratory with the proper permits to test for the presence of R. solanacearum race 3 biovar 2.
If detection of R. solanacearum race 3 biovar 2 is confirmed in geraniums, the current guidelines prescribe immediate suspension of production, traceback and traceforward investigations, and required destruction of infected, or potentially infected, varieties or lots. All associated host material that share the same irrigation system or unsanitary greenhouse practices, are also candidates for destruction. All production areas, greenhouse articles, soil, and water systems must be disinfested according to agency guidelines prior to the release of a facility or growing area.
| References |
APHIS-PPQ. 2004. Minimum sanitation protocols for offshore geranium cutting production. 27 p. Published Dec., 1. USDA, APHIS, PPQ. Pest Detection and Management Programs, Riverdale, M. D.
CABI/EPPO. 1999. Distribution maps of plant diseases. Map N0. 785 CAB International. Wallingford, U. K.
Coutinho, T. A. 2005. Introduction and prospectus on the survival of R. solanacearum. Pages 29-38 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. Allen, C., Prior, P., and Hayward, A. C., eds. APS press, St. Paul, M. N.
Denny, T. P. 2006. Plant pathogenic Ralstonia species. Pages 573-644 in: Plant-associated bacteria. S. S. Gnanamanickam, ed. Springer Publishing, Dordrecht, The Netherlands.
Elphinstone, J. G. 2005. The current bacterial wilt situation: a global overview. Pages 9-28 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. C. Allen, P. Prior, and A. C. Hayward, eds. APS press, St-Paul, M. N.
Fegan M. and Prior P. 2005. How complex is the "Ralstonia solanacearum" complex ? Pages 449-461 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. C., Allen, P., Prior, A. C., Hayward, eds. APS Press, St. Paul, M. N.
Floyd, J. 2003. Action plan for Ralstonia solanacearum race 3, biovar 2 found in nursery facilities. 23 p. Published Feb., 27, Version 3. USDA, APHIS, PPQ. Pest Detection and Management Programs, Riverdale, M. D.
Floyd, J. 2004. New pest response guidelines. Ralstonia solanacearum race 3 biovar 2. Southern wilt of geranium. Published Jan., 14, Version 4.0. USDA, APHIS, PPQ. Pest Detection and Management Programs, Riverdale, M. D.
Harmon, P. F., Harmon, C. L., Norman, D., Momol, T. 2005. Southern wilt of geranium. 5 p. University of Florida, Cooperative Extension Service, Institute of Food and Agricultural Sciences, Florida, U. S. Fact Sheet PP-206.
Lambert, C. D. 2002. Agricultural bioterrorism protection act of 2002: possession, use, and transfer of biological; agents and toxins; interim and final rule. (7 CFR Part 331). Federal Register 67:76908-76938.
Saddler, G. S. 2005. Management of bacterial wilt disease. Pages 121-132 in: Bacterial wilt disease and the Ralstonia solanacearum species complex. Allen, C., Prior, P., and Hayward, A. C., eds. APS press, St. Paul, M. N.
| Author: | Patrice G. Champoiseau of University of Florida |
| Reviewers: | Caitilyn Allen of University of Wisconsin; Jeffrey B. Jones, Carrie Harmon and Timur M. Momol of University of Florida |
| Publication date: | September 12, 2008 |
| Supported by: | The United States Department of Agriculture - National Research Initiative Program (2007-2010) |

